 Clomiphene citrate is prescribed for:
Clomiphene citrate is prescribed for:
Increasing the chance of pregnancy by helping women ovulate (produce a mature egg) during their cycle
Increased testosterone production in males
Inhibiting the effects of estrogen in males
Dosage strength of Clomiphene available:
25 mg Tablet
50 mg Tablet
100 mg Tablet
Pharmacological Classifications: Selective Estrogen Receptor Modifier(SERM), Fertility Agent
General Information
Clomiphene is a nonsteroidal fertility agent used to induce ovulation in infrequently ovulating or anovulatory women, including patients with polycystic ovary syndrome (PCOS). The drug is effective at producing ovulation in patients with an intact hypothalamic-pituitary-ovarian axis and with ovaries which are capable of functioning. Clomiphene is the first-line agent for all these patients because of the relative ease of use and low economic expense. Ovulation will be induced by clomiphene in 80% of -chosen patients; roughly 40% will become pregnant within 6 cycles of treatment. Clomiphene isn’t effective at increasing pregnancy rates. Clomiphene therapy is associated with only slightly increased rates of multiple births (3-5%) compared to the general population (1%). Multiple gestation, if it occurs, is twins. Less than 1% deliver more or triplets. The rate of multiparity with clomiphene as a single-agent is much lower than that which occurs with other fertility agents (e.g., menotropins or FSH). Clomiphene is used as a diagnostic tool to assess ovarian reserve. In those patients receiving donor insemination, it’s administered to regulate the timing of ovulation. Clomiphene was used to increase sperm counts in male patients with idiopathic oligospermia. Clomiphene was approved by the FDA in 1967.
Clomiphene Citrate is a medicine used for female patients unable to get pregnant due to ovulation problems. This medicine helps to stimulate healthy ovulation, which is especially helpful in cases when the patient’s ovaries are unable to produce an egg due to deficient hormonal stimulation. You can buy Clomiphene Citrate over the counter online easily, as long as you have a reliable pharmacy to go to.
Medical uses
The main purpose of use is to treat female infertility, which is achieved by stimulating the production of a specific hormone in the body. Another possible use for Clomiphene Citrate includes bodybuilding, as it helps the athletes keep their hormone levels at healthy levels while training. However, this is an off-label use for Clomiphene Citrate than always needs to be discussed with a qualified healthcare professional.
Available forms
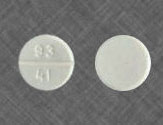
Clomiphene Citrate comes as a white scored tablet that contains 50 mg of the active ingredient. Also available Clomiphene 25 mg and 100 mg. 50 mg is usually a starting dose for most patients. If the patient requires a higher dose, two tablets of Clomiphene Citrate are prescribed and so on. However, higher dose of Clomiphene Citrate can also cause more side effects, which is something to take into account.
Mechanism of Action
Clomiphene was recently re-classified as a selective-estrogen-receptor-modulator (SERM) because of its capability to compete with estradiol for estrogen receptors in the level of the hypothalamus. Clomiphene blocks the typical negative comments of circulating estradiol on the hypothalamus, preventing estrogen from reducing the out-put of gonadotropin-releasing hormone (GnRH). During treatment, amplitude and the frequency of GnRH pulses improve and promote the pituitary gland to produce FSH and LH. Consequently, LH and FSH stimulate follicles to be developed by the ovaries. Clomiphene might permit oocytes to develop to maturity in a few patients. Estrogen made by the follicles that are maturing causes the mucus to proliferate to the endometrium as well as thin. As the estradiol from these follicles rises, the positive-feedback to the pituitary causes the FSH/LH surge and is accompanied by by mature oocyte release (ovulation). The corpus luteum types once ovulation occurs, and levels rise as they prepare the endometrium for implantation, and do in a normal ovulatory cycle. Clomiphene has to be used prior to time of typical choice of the dominant follicle in a ovulatory cycle. The “conventional” timing of clomiphene therapy is from follicular stage cycle day 5 through 9; females usually ovulate 5-10 times after the last dose of the cycle. However, earlier initiation of therapy, longer programs (i.e., up to 10 times), or or more clomiphene doses might be required dependent on the ovulatory response of the individual girl.
Clomiphene is a weak estrogen-agonist and can interact with estrogen receptor containing tissues, for example, ovary. Clomiphene can interfere together with the capacity of mucus glands to create secretions that favor conception and sperm motility. In a few women, dry, thick secretions may be induced by clomiphene. Studies are continuing to evaluate the the experience of clomiphene. The effects of clomiphene are secondary to the main results on hypothalamic-pituitary-ovarian function. Clomiphene displays no androgenic, anti-androgenic, or effects, nor does it impact -thyroid or pituitary- function. After treatment discontinuation, there’s usually no ongoing pharmacological influence on subsequent cycles. However, in a few females ovulation h-AS continued.
In female individuals that were selected, other drugs augment clomiphene therapy to boost the event of pregnancy or effective ovulation. These medicines have included: dexamethasone for hirsutism or hyperandrogenism; estradiol to offset the cervical mucus-thickening result of clomiphene; human chorionic gonadotropin (HCG) to induce the LH-surge for ovulation; and also the use of metformin or troglitazone to off-set the hyperinsulinemia that does occur in some patients with poly-cystic ovaries. Octreotide continues to be used to clomiphene therapy inpatients with polycystic ovaries de-crease LH ranges so that you can reduce androgen secretion, and possibly de crease the incidence of ovarian hyper-stimulation. The mechanisms of motion of those drugs don’t seem to modify the pharmacokinetics of remedy and are unique from clomiphene. The regimens of every one of these drugs that are augmenting demands cautious monitoring and timing of administration underneath the prescription of a doctor experienced in endocrinology and fertility.
In males with idiopathic oligospermia, clomiphene could be successful a-T stimulating GnRH re lease from your hypothalamus, ergo growing the ranges of FSH and LH introduced in the pituitary. FSH and lH seemingly promote the testicles to create sperm and testosterone. Density and sperm quantity are improved; nevertheless, clomiphene might perhaps not boost motility or sperm maturation.
Pharmacokinetics
Clomiphene is administered. Clomiphene is a mixture of isomers, zuclomiphene and enclomiphene, which may possibly have diverse pharmacodynamic and pharmacokinetic parameters that haven’t been completely elucidated. Zuclomiphene is believed to be the mo-Re estrogenic isomer. The drug seems to to endure hepatic k-calorie burning, although the destiny of clomiphene h-AS not been entirely proven. Both drug and its own metabolites are excreted in the feces through the bile. Stereo- sequestering or certain enterohepatic recycling appears to to happen with the isomer. However, in spite of cycles of use, blended maximum plasma ranges of zuclomiphene and enclomiphene don’t seem to surpass 100 mmol/L. The halflife of clomiphene is noted to be 5 times, but studies with doses have shown the drug could possibly be present in the feces for up to 6 months.
Route-Particular Pharmacokinetics
Oral Route: Clomiphene is well-absorbed in the GI tract.
Indications
Clomiphene Citrate is commonly recommended to improve a woman’s probabilities of pregnancy by improving ovulation- the capacity to make a mature egg throughout the menstrual period. In guys, it functions to improve testosterone creation, also to equally hinder the outcomes of estrogen. As anti-estrogen, clomiphene citrate functions in males, and could possibly be utilized to counter a few of along side it outcomes of steroid use including elevated water-retention and gynecomastia. As an anti-estrogenic medication, it could also generate an elevation of LH and FSH, which boost sperm manufacturing and can elevate testosterone.
Contraindications and Precautions
Your health care provider needs to know for those who have these conditions: Adrenal gland disease; blood vessel disease or blood clots; cyst on the ovary; endometriosis; liver disease; ovarian cancer; pituitary gland disease; vaginal bleeding that has not been evaluated; an unusual or allergic reaction to clomiphene, other medicines, foods, dyes, or preservatives; pregnant (shouldn’t be used if you’re already pregnant); breast-feeding
Clomiphene is contraindicated in patients that are hypersensitive to the drug or the components of the dosage form for use.
Clomiphene will be effective at inducing ovulation in women using an intact hypothalamic- ovarian and pituitary tract response. Patients with pituitary insufficiency untreated adrenal insufficiency or primary pituitary failure, pituitary adenoma or alternative pituitary tumor; primary ovarian failure; or untreated thyroid disease WOn’t respond to clomiphene treatment.
Reduced responses to clomiphene therapy may occur in a few females with diabetes mellitus, hirsutism (or hyperandrogenism), hyperinsulinemia, obesity, or lowered levels of endogenous estrogen. Sometimes, the use of other medications with clomiphene may increase the chances of successful conception.
In men, clomiphene has been effective for treating idiopathic oligospermia. Men including primary testicular failure or disruption of hypothalamic-pituitary function, aren’t expected to respond to clomiphene treatment.
Clomiphene is contraindicated for use in patients with dysfunctional or abnormal uterine bleeding of undetermined origin. Neoplasm (e.g., ovarian cancer or endometrial cancer) should be ruled out before clomiphene use. Because clomiphene may stimulate endometrial growth, it needs to be used cautiously in patients with uterine leiomyomata (fibroids) or endometriosis. It is often suggested the use of fertility drugs, including clomiphene, might increase the danger of developing certain cancers. A number of case reports along with some observational studies gave rise to the speculation the increased number of ovulatory follicles and/or high gonadotropin and estrogen levels might enhance the development of ovarian cancer or breast cancer. However, infertility is a completely independent risk factor for the development of ovarian or breast cancer. Case reports control for other independent confounding factors including delay in family or parity history. In one long-term cohort study of 1, 197 infertile women, the incidence of breast or ovarian cancer wasn’t elevated in the groups receiving fertility treatments versus those. The breast cancer rate, in particular, wasn’t significantly different in either group versus the general population that is female. Current data don’t support an association involving the use of fertility drugs and increased cancer risks while more studies are needed.
A full gynecologic exam ought to be performed. Clomiphene shouldn’t be administered to patients with ovarian enlargement or a preexisting ovarian cyst that’s not due to polycystic ovarian syndrome (PCOS). Clomiphene therapy shouldn’t be initiated until the diagnostic cause of enlargement or the cyst continues to be determined and ovary size has returned to normal. The lowest dose must be used to minimize the chance of either uncomplicated ovarian enlargement or OHSS in any female. There isn’t any advantage to increasing the dose once an ovulatory dose continues to be determined. Some patients with polycystic ovary syndrome (PCOS) are unusually sensitive to gonadotropins and may have an exaggerated response to clomiphene treatment; lower dosages could be needed. All female patients needs to be instructed to report symptoms of ovarian enlargement, including abdominal pain or pelvic pain; nausea; vomiting; ascites (fluid and distension in the abdomen); or weight gain immediately. The current cycle of clomiphene ought to be halted if cysts develop or if ovarian enlargement or OHSS occurs; maximal ovarian enlargement may not be evident until several days. Clomiphene therapy shouldn’t be reinstated until ovary size has returned to normal. A complete pelvic exam needs to be repeated in most female patients prior to every treatment cycle.
Clomiphene is contraindicated in those patients using a history of dysfunction or hepatic disease. Reduced clearance of clomiphene might increase the danger of certain side effects and may result in higher plasma concentrations. Moreover, hepatotoxicity has occurred in a few patients together with ovarian hyperstimulation syndrome (OHSS). Determinations of liver function ought to be performed ahead of clomiphene therapy in most patients.
Clomiphene ought to be used cautiously in patients with psychosis or major depression. There exists a chance the status could be exacerbated. Depression may be common in the infertile population because of the experience of loss present in cycles in.
Clomiphene needs to be used cautiously in patients with other active thromboembolic disease or active thrombophlebitis. Like other selective estrogen receptor modifiers (SERMs), clomiphene exhibits some estrogenic activity. Thromoembolic events are reported in patients.
Patients may be made by clomiphene or else they may have blurred vision. They are affected by caution patients against driving or operating machinery, or performing tasks that need mental alertness, until they’re aware. Any visual disturbance should be promptly reported by the patient for their health care prescriber, and an ophthalmologic examination ought to be performed.
Drinking alcoholic beverages, including ethanol intoxication, or tobacco smoking are two lifestyle choices which will decrease the effectiveness of fertility treatments or fertility in and/or a few women men. While pursuing fertility therapies like those patients should avoid excessive alcohol or tobacco consumption.
Pregnancy
Clomiphene is contraindicated for use after conception has occurred, and is classified as FDA pregnancy risk category X. There aren’t any adequate human studies of the effects of clomiphene on the fetus. Animal reproduction studies have shown adverse fetal effects for example, development of ocular defects and congenital structural bladder and bowel changes, in early gestation. Clomiphene doesn’t appear to increase the risk of spontaneous abortion or congenital abnormalities . Data suggest that teratogenesis might occur if clomiphene is continued in early pregnancy. Adequate evaluation of the patient is necessary before a subsequent clomiphene therapy course is undertaken to make sure that pregnancy hasn’t occurred carrying out a course of clomiphene. As well as potential effects on the fetus, clomiphene use does increase the danger of multiple gestation as well as the risks inherent with such pregnancies.
Breast-Feeding
It’s not known whether clomiphene is excreted in human milk. Because many drugs are excreted in human milk, caution ought to be exercised if clomiphene is administered to your woman who’s breast-feeding. In certain patients, lactation may be reduced by clomiphene; several studies found that it suppresses lactation in women who didn’t want to breast-feed. The mechanism might be due to decreases in serum prolactin, especially the post-stimulation surge in serum prolactin. It’s likely that clomiphene would interfere with lactation in a nursing mother. If clomiphene therapy is necessary in the mother alternate forms of feeding for the infant will be recommended.
Interactions
In women, androgens may antagonize the effects of some fertility treatments. Prasterone DHEA is converted via hydrosteroid dehydrogenases and aromatase into testosterone, androstenedione, and estradiol by peripheral tissues. Increased endogenous levels of the hormone prasterone, dehydroepiandrosterone, DHEA happen to be associated with infertility and hyperandrogenism; it’s postulated that nutritional supplementation with DHEA may reduce the response.
Some alternative or herbal therapies may have effects on hypothalamic-pituitary function or hormone concentrations and thus may interfere with fertility therapies. Chaste tree fruit, Vitex agnus-castus may interfere with all the therapeutic activity of infertility drugs. A case of multiple follicular development and ovarian hyperstimulation syndrome continues to be reported in a woman who took prior to one of her IVF protocol cycles; hormone levels and serum gonadotropin were disordered and pregnancy wasn’t accomplished. As a result of lack of data, herbal therapies touted for the health of women aren’t recommended for use with fertility treatments that were prescribed.
Black cohosh, Cimicifuga racemosa, is a phytomedicinal that may potentially suppress luteinizing hormone (LH). 7 8It could potentially certain. As a result of lack of data, any herbal therapies touted for the health of women aren’t recommended for use with fertility treatments that were prescribed.
The soy isoflavones may compete. Soy isoflavones needs to be used with caution in patients.
Adverse Reactions/Side effects
Clomiphene Citrate may cause side effects in some patients, such as breast tenderness, abdominal tenderness or swelling, vomiting, headache, abdominal or pelvic pain, breast discomfort, nausea, abnormal uterine bleeding, visual disturbances, flushing and diarrhea. All those need to be reported to your doctor to see how they should be dealt with. In most cases, you will be able to buy Clomiphene Citrate and take in spite of those symptoms, because one cycle takes only 5 days, after which the symptoms are likely to go away.
Clomiphene therapy is associated with only slightly increased rates of multiple births (3-5%) compared to the general population (1%). Over 90% of women on clomiphene therapy experience gestations that are single. Multiple gestation, if it occurs, is twins. Less than 1% deliver even more or triplets. The rate of multiparity with clomiphene as a single-agent is typically much lower than that which occurs with other fertility agents (e.g., menotropins or FSH).
Clomiphene therapy is well tolerated, and side effects don’t commonly interfere together with the continuation of treatment. Many patients experience vasomotor “hot flashes” (10.4%), which result from the anti-estrogen effects; these subside at the completion of each 5 day dosage cycle. Mild pelvic discomfort without enlargement of the ovaries occurs in roughly 5.5%. Breast tenderness/mastalgia was reported in 2.1% of patients. Less frequent side effects include dizziness (less than 1%), fatigue (less than 1%), and headache (1.3%). Abnormal uterine bleeding, reported as menorrhagia or breakthrough bleeding, occurred in 1.3% of patients. Menstrual irregularity and an increase in pain associated with ovulation (mittelschmerz) may occur (less than 1.5%).
The normal thinning of the cervical mucus in preparation for conception and ovulation might be impaired by clomiphene therapy. Cervical mucus could decrease the ability of sperm to make it to the oocyte and may result in up to 25% of patients taking clomiphene. Vaginal dryness may occur. Although controversial, estrogens are administered in a few patients on cycle day 10 to time of the LH surge (ovulation) so as to combat mucus drying and thickening. There’s little documentation of the efficacy of estrogen in pregnancy rates that are improving.
Roughly 14% (1 out of every 7 women) on clomiphene therapy experience uncomplicated ovarian enlargement. Pelvic examinations needs to be performed in patients who complain of abdominal discomfort (pelvic pain) during therapy. If cysts or enlargement are present, the current clomiphene cycle needs to be discontinued and further therapy withheld until resolution of symptoms and the signs and before the ovary is enlarged. Use the lowest dose consistent with clinical results that are expected to minimize the hazard associated with occasional abnormal ovarian enlargement. Maximal enlargement of the ovary may not occur until several days after discontinuation of the clomiphene dose that is recommended. Sexual intercourse ought to be prohibited due to the threat of hemoperitoneum secondary if substantial ovarian enlargement occurs after ovulation. Adjunctive agents (i.e., human chorionic gonadotropin, HCG) should be withheld if ovarian enlargement is present in order to prevent progression to ovarian hyperstimulation (OHSS). Most ovarian cysts or enlargements will regress in just a day or two to weeks after discontinuation of clomiphene. Laparoscopy is needed. The dosage or duration of the next cycle of clomiphene ought to be reduced once recovery occurs. Ovarian cyst formation or ovarian enlargement may be more likely to occur in patients with polycystic ovary syndrome or in those patients on higher dosages that are cyclic, receiving a lot more than six total cycles of therapy, or receiving treatment beyond 5 days in every single cycle.
Abdominal pain, nausea/vomiting (2.2%), acute abdomen (less than 1%), constipation (less than 1%), diarrhea (less than 1%), and weight gain (less than 1%) have been noted with clomiphene. Of note, a few of the early warning signs of the ovarian hyperstimulation syndrome are abdominal pain and distention, nausea, vomiting, diarrhea, and weight gain. Instruct the patient to report weight gain, any abdominal or pelvic pain, discomfort, or distention.
Clomiphene therapy can result in ovarian hyperstimulation syndrome (OHSS). The incidence of OHSS with clomiphene is less than that which occurs together with the administration of menotropins or follicle-stimulating hormone. OHSS is a medical event distinct from uncomplicated ovarian enlargement and requires inpatient care. The syndrome could be severe. The pathogenesis of the syndrome is still unknown, but probably results in the production and secretion of many substances (i.e., prostaglandin, cytokines, vascular endothelial growth factor, and activation of the ovarian-renin-angiotensin system) in response to the stimulation of ovulation. Patients have exhibited increased plasma angiotensin-converting enzyme (ACE) activity in association with this particular syndrome. Some early warning signs include distention and severe abdominal pain, pelvic pain, nausea/ diarrhea, vomiting, and weight gain. Severe cases produce clinical signs including gastrointestinal symptoms, gross ovarian enlargement, ascites, dyspnea, oliguria, and pleural effusion. Other reported symptoms of OHSS include pericardial effusion, anasarca, hydrothorax, acute abdomen, elevated hepatic enzymes, hypotension, renal failure (unspecified), pulmonary edema, intraperitoneal and ovarian hemorrhage, deep venous thrombosis, stroke, torsion of the ovary, and acute respiratory distress. Hypoalbuminemia, electrolyte imbalance, hypovolemia, hemoconcentration, and elevated urinary steroid levels may occur. Hypovolemic shock hemoconcentration, or thrombotic events may be fatal. Abdominal and pelvic examination needs to be done because of the fragility of the enlarged ovaries in severe cases of OHSS. Conception may result in progression. Clomiphene therapy shouldn’t be reinstated until ovary size has returned to normal.
Clomiphene can cause treatment-associated visual impairment in roughly 1.5% of patients. The incidence may increase with increasing dosage, although these events are infrequent. Vision changes require that the prescriber is notified by the patient. Adverse effects include blurred vision, diplopia, scintillating scotomata (spots and flashes), and photophobia. Macular edema, abnormal accommodation, cataracts, ocular pain, postmarketing, optic neuritis, photopsia, posterior vitreous detachment, retinal hemorrhage, retinal vascular spasm, and temporary lack of vision have already been reported. You can find rare reports of temporary but severe diminished visual acuity (blindness). Visual symptoms are accentuated upon contact with bright light. These effects can impair a patient’s ability. Clomiphene therapy ought to be discontinued if visual disturbances occur, and complete ophthalmologic testing ought to be performed. Generally, clomiphene induced visual changes are reversible within several days of drug discontinuation.
Dermatological adverse reactions to clomiphene therapy include dermatitis or rash (unspecified) (less than 1%) and alopecia (less than 1%). Allergic dermatitis, erythema multiforme, urticaria, pruritus, acne vulgaris, erythema, erythema nodosum, hypertrichosis, and postmarketing happen to be reported.
Central nervous system (CNS) adverse effects don’t happen frequently (less than 1% of patients using clomiphene). CNS adverse effects include insomnia, depression, anxiety, lightheadedness, and restlessness. Irritability, migraine, psychosis, emotional lability, and postmarketing happen to be reported.
Rare side effects occurring using clomiphene include weight loss, increased urinary frequency, and appetite stimulation.
Postmarketing, pulmonary embolism, thrombo-phlebitis, retinal thrombosis, arrhythmia, chest pain (unspecified), edema, hypertension, palpitations, dyspnea, and sinus tachycardia have been reported with clomiphene. Rare thromboembolic events, like phlebitis, arterial occlusion, and pulmonary embolism happen to be reported with clomiphene therapy in women that have not had ovarian hyperstimulation that was concurrent. The association of clomiphene with all the development of thromboembolic disease isn’t clear. Certain patients with other risk factors for thromboembolism may be at higher risk.
Prolonged use of clomiphene may increase the danger of a borderline or invasive ovarian tumor, although a causal relationship between ovarian cancer and ovarian hyperstimulation hasn’t been determined. Perform a thorough evaluation if ovarian cysts don’t regress spontaneously. Various cancers happen to be noted including but not limited trophoblastic, and neoplasms. Some observational studies along with a number of case reports gave rise to the speculation that infertility treatments might enhance the danger of secondary malignancy in women (i.e, breast cancer or ovarian cancer). However, infertility is a completely independent risk factor for the development of ovarian or breast cancer. Observational studies and case reports suggesting an association of cancer to fertility treatments control for other independent confounding factors including delay in family or parity history. In one long-term cohort study of 1, 197 infertile women, the incidence of breast or ovarian cancer wasn’t elevated in the groups receiving fertility treatments versus those. The breast cancer rate, in particular, wasn’t significantly different in either group versus the general population that is female. While more studies are needed, the current data don’t support an association between the use of increased cancer risk and fertility drugs.
Clomiphene doesn’t appear to increase the risk of spontaneous abortion or congenital abnormalities . The overall incidence of reported birth anomalies from pregnancies associated throughout the investigational studies with maternal clomiphene ingestion was within the number of the reported in published references for the general population. Data suggest if clomiphene is continued in early pregnancy that teratogenesis might occur during organogenesis. Numerous congenital malformations happen to be reported postmarketing. Animal studies reveal a possible connection of clomiphene use with bowel syndromes and congenital ocular or other structural malformations. It’s important that clomiphene therapy be continued in subsequent fertility treatment cycles only after pregnancy continues to be ruled out and normal withdrawal bleeding (menses) has occurred.
Postmarketing, hepatitis and elevated hepatic enzymes happen to be noted with clomiphene. Transient liver function test abnormalities suggestive of hepatic dysfunction which could be accompanied by morphologic changes on liver biopsy happen to be reported in association using the ovarian hyperstimulation syndrome.
This list may not include all possible adverse reactions or side effects. Call your health care provider immediately should you be experiencing any signs of an allergic reaction: skin rash, itching or hives, swelling of the face, lips, or tongue, blue tint to skin, chest tightness, pain, difficulty breathing, wheezing, dizziness, red, a swollen painful area/areas on the leg.
How is this medication best taken?
Take this medicine by mouth using a glass of water. Follow the directions on the prescription label. Take as directed for the exact number of days. Take your doses at fixed intervals. The period of treatment might be adjusted, although most women take this medicine to get a 5 day period. Your doctor can give you instructions on proper use and will provide you with a start date with this medication. Don’t take your medicine more often than instructed. Talk to your own pediatrician about the use of the medicine in children. Special care could possibly be needed. Overdosage: In The Event you think you’ve got taken too much with this medicine contact emergency room or a poison control center simultaneously. NOTE: This medicine is for you personally. Don’t share this medicine with others.
What do I do if I miss a dose?
In the event you miss a dose, take it. When it is almost time on your next dose, forget the missed dose. Take your next regularly scheduled dose. Don’t take two doses in the same time.
Storage
Keep from the reach of children. Store at room temperature between 15 and 30 degrees C (59 and 86 degrees F). Protect from light, heat, and moisture. Throw away any unused medicine following the expiration date. DON’T FLUSH UNUSED MEDICATIONS OR POUR DOWN A SINK OR DRAIN.
General Statements
Don’t share or take the medicine of any one else. Talk along with your healthcare provider before starting any new medicine, including over-the-counter, natural products, or vitamins. This medication was compounded for you personally. This patient information summarizes the most important information about your medication; talk by means of your doctor if you’d like more information.